Department of Parasitology and Tropical Medicine (4)
National consulting office for parasitic and tropical diseases
HEAD OF THE OUTPATIENT’S CONSULTING OFFICE:
ISKREN KAFTANDJIEV, MD
E-MAIL: This email address is being protected from spambots. You need JavaScript enabled to view it.
NCIPD Consulting office:
Sofia, Krakra Street № 3-A
Consulting office hours: Monday – Friday, from 8 a.m to 3 p.m.
Staff of the Consulting office:
Svetla Popova – hygiene assistant
Outpatient’s unit for consulting assistance in the Department of Parasitology and Tropical Medicine at the NCIPD is performing the following basic activities: consultations, diagnostic activities, therapeutic activities, follow up observation, tutoring and scientific research.
The consulting room is performing medical services for patients from Sofia and the country, who had been directed for specialized assistance in the field of medical parasitology from other health facilities, or have decided to attend for consultation on their own. An advisory help is provided for travelers in tropical countries regarding the risk from tropical diseases, the ways of prevention and the necessity of chemoprophylaxis. The following specialized and common health services are performed:
1. Medical examination and consultation:
- Primary examination with anamnesis and physical patient’ status evaluation.
- Secondary examination and evaluation of a new medical problem.
- Switching patients for other qualified assistance with specialists in outpatients’ cabinets or for hospital services.
- Medical examination and consultation of traveling persons coming from or departing for tropical countries.
- Issuing of medical documentation.
- Issuing expert decision for NHIF/RHIF regarding the necessity of anti-relapsing and/or conservative treatment of cystic echinococcosis.
- Diagnostic specification, follow up observation, and therapy of pregnant mothers with acute toxoplasmosis.
- Examination and consultation of hospitalized patients at hospital facilities.
2. Medical manipulations:
- Injections.
- Taking blood samples for microscopic and serological examination.
- Peripheral blood sampling for malaria examination.
- Cutaneous samples from skin lesions for leishmaniosis.
- Pathological specimen collection from urethra and vagina for parasitological and microbiological examination.
- Perianal cellophane-tape sampling.
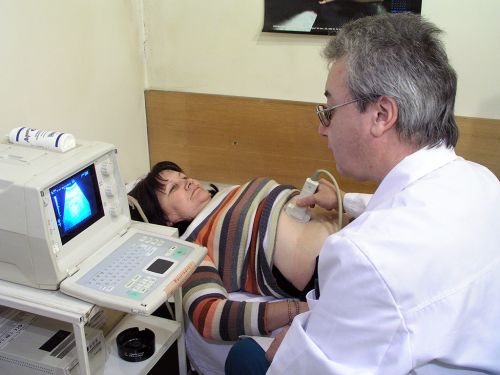
3. Diagnostics:
- Macroscopic examination for parasites.
- Wet mount preparations for parasites.
- Abdominal Ultrasound Sonography.
4. Follow up observation
Follow up monitoring of different nosological units (parasitological diseases) for different category patients - children and adults is performed, according to the Ministry of Health regulations. Patients suspected for, or with confirmed echinococcosis, are included in a card - index for continuous dispensary monitoring.
5. Education
Outpatient’s office specialists are actively participating in educational activities held in the department (in the form of individual training or courses), which are included in the program for post-graduate education in Medical Parasitology, according to an approved NCIPD program.
Individual training and courses on issues of the clinical aspects, contemporary therapy, follow up observation and prophylactic treatment of indigenous and imported parasitoses are annually held in the department. Тhe courses are intended for specializing physicians, parasitologists, general practitioners, physicians from other clinical disciplines - internists, pediatricians, gastroenterologists, infectionists and others.
6. Scientific and Research activities
These activities are represented by the numerous participations in scientific works /one-man or complex with the participation of other scientific units/, publications in scientific magazines, author issues of monographs and manuals, participation in national and international forums. The scientific research is mainly in the field of clinics, experimental and clinical chemotherapy, dispensary observation, distribution and chemotherapy of indigenous and imported from tropical countries parasitoses. Of scientific interest are parasitological diseases like echinococcosis, leishmaniasis, trichinellosis, toxoplasmosis, toxocarosis, trichomonasis, giardiasis, dirofilarioses, cysticercosis and others.
Written by Super UserLaboratory of zooantroponozes and vector-borne parasitoses

Head: Prof. Iskra Rainova, MD, DSc
E-mail: This email address is being protected from spambots. You need JavaScript enabled to view it.
Laboratory staff:
- Ognyan Mikov, PhD, E-mail: This email address is being protected from spambots. You need JavaScript enabled to view it.
- Eleonora Kaneva, PhD, E-mail: This email address is being protected from spambots. You need JavaScript enabled to view it.
- Genka Toteva - medical technician
- Nadya Kamenova - laboratory help

The main activities of the laboratory staff fall within the following areas:
- Obtaining and characterization of antigens from some parasitic species: Toxoplasma gondii, Trichinella spiralis, Leishmania spp., Entamoeba histolytica
- Development of bioproducts for serological diagnostics and culture media for some parasitic diseases such as:
- ELISA test kits for the detection of IgG antibodies in echinococcosis, trichinellosis, toxoplasmosis
- Indirect haemagglutination (IHA) tests for toxoplasmosis and trichinellosis
- Culture media for the cultivation of Trichomonas vaginalis, Entamoeba histolityca, and Blastocystis hominis.
- Immune rabbit sera against Toxoplasma gondii, Echinococcus granulosus, and Trichinella spiralis.
- The research work of the laboratory staff is directed towards studying the possibilities for the application of new serological methods for the diagnosis of parasitoses and analysing the impact of the parasites on the human immune system. Members of the laboratory participate in the implementation of four scientific projects financed by the Scientific Research Fund:
- "Studies on the cellular and humoral immune response in patients with acute and chronic form of toxoplasmosis", KP-06-N43/2 of 27.11.2020.
Duration: 36 months
Project leader: Prof. Iskra Raynova, MD, DSc
- "Biological and molecular genetic studies on the insecticide resistance of native and invasive mosquito species", KP-06-N41/5 dated 30.11.2020.
Duration: 36 months
Project leader: Assistant Professor Ognyan Mikov, PhD
- "Phylogenetic and genotypic analysis of Enterobius vermicularis and studies on the influence of the parasite on the local immunity of the intestinal tract and the possibility of bacterial co-infections in infected persons in Bulgaria", KP-06-PN53/4 of 11.11.2021.
Duration: 36 months
Project leader: Assistant Professor Eleonora Kaneva, PhD
- "Caves as a reservoir of new and re-emerging zoonoses - ecological monitoring and metagenomic analysis in real time", KP-06-N51/9 dated 11/16/2021.
Duration: 36 months
Project leader: Associate Professor Nikolay Simov, PhD from the National Museum of Natural History - BAS
- Educational activity is carried out according to the programme for postgraduate training, organised in NCIPD.
- Experts of the laboratory also are involved in the preparation of the annual analysis of parasitic morbidity in the country, development of national programs and research projects.
Written by Super User
NATIONAL REFERENCE LABORATORY (NRL) for Diagnosis of Parasitic Diseases
NATIONAL REFERENCE LABORATORY (NRL) for Diagnosis of Parasitic Diseases
Working hours: Monday to Friday from 7:30 to14:30
Head: Associate Professor Nina Dimitrova Tsvetkova, PhD
E-mail: This email address is being protected from spambots. You need JavaScript enabled to view it.
Phone: +359 2 843 80 02; +359 2 94 46 999/ 316; 329; 311
Fax: +359 2 843 80 02
Laboratory staff:
Raina Borisova, MD
Aleksandra Ivanova, biologist,
Mihaela Vanyova Videnova, biologist
Violeta Chavdarova Yakimova, biologist
Galina Mesdraliiska, technical assistant
Silvia Koleva – laboratory worker
The Ministry of Health (MH) has attributed to the laboratory the official status of National Reference Laboratory for the Diagnosis of Parasitic Diseases (NRLDPD). The laboratory is in charge of the organization and implementation of a National System for External Quality Assurance (EQA) of parasitic laboratory diagnosis carried out by all parasitological laboratories in the country. NRLDPD is included in the German Scheme of Quality Assurance - Instand. The laboratory has obtained accreditation status according to the standard EN ISO/IEC 17025:2001. Reference diagnosis of malaria, toxoplasmosis, leishmaniasis, amebiasis, trichinellosis, echinococcosis, pneumocystosis, toxocarosis is performed.
All parasitological laboratories and clinical departments in the country are provided with technical assistance. NRLDPD is also in help of the MH and Regional Health Inspectorates in cases of outbreakes and epidemics. Epidemiological work and annual analysis of the parasitic morbidity is conducted as well as evaluation of the activities of the parasitological network in the country. The laboratory staff is involved in postgraduate education.

The laboratory carries out routine and reference diagnosis by applying contemporary laboratory methods – morphological, immunological and biomolecular.
1. Intestinal helminthic diseases: Аscariasis, Trichuriasis, Enterobiasis, Тaeniasis, Hymenolepiasis, Strongyloidiasis, Ancylostomiasis, Diphyllobothriasis, Fascioliasis, Dicrocoeliasis, intestinal Schistosomiasis and other rare or tropical helminthic diseases. Material: stool
- Маcroscopic examination and morphological differentiation of intestinal helminths
- Microscopic examination for helminth eggs and larvae:
- Wet mount preparations – with saline and iodine solutions
- Concentration procedures (sedimentation, flotation, formalin - ethyl acetate sedimentation technique)
- Detection and larvae cultivation - Baermann funnel technique, Harada-Mori filter paper technique
- Transparent tape test: Enterobiasis and Taeniasis (T. saginata)
- Immnodiagnostic methods in Fascioliasis (ELISA, IHA, IFA)
2. Intestinal protozoal diseases (Giardiasis, Amebiasis, Balantidiasis, Blastocystis infection, Cryptosporidiosis, Cystoisosporiasis, Cyclosporiasis, Мicrosporidiosis). Materials: stool, duodenal material (Giardiasis), biopsy material:
- Мicroscopic examination for trophozoits, cysts and oocysts:
- Wet mount preparations – with saline and iodine solutions
- Concentration procedure – formalin - ethyl acetate sedimentation technique
- Staining procedures – Giemsa, trichrome, modified Ziehl Neelsen staining, etc.
- Cultivation: Pavlova’s medium (Аmebiasis, Blastocystis infection)
- Immunodiagnostic methods in Amebiasis (ELISA)
- Biomolecular methods: PCR for Giardiasis, Blastocystosis, Cryptosporidiosis
3. Blood and tissue parasitic diseases
Мalaria and Babesiosis. Materials: peripheral (capillary) blood for thick and thin blood films, Rapid Diagnostic Tests and PCR
- Microscopic examination: Staining procedures - Giemsa stain
- Biomolecular methods: PCR for malaria species identification
Visceral leishmaniasis. Materials: bone marrow and serum for immunodiagnostics, as well as biopsy materials from lymph node, liver, and spleen.
- Microscopic examination:
- Staining procedures - Giemsa stain
- Cultivation techniques: cultivation in Novy-MacNeal-Nicolle (NNN) medium
- Immunodiagnostic methods – ELISA, Western blot
- Biomolecular methods – PCR for diagnostics and species identification
Cutaneous and mucocutaneous leishmaniasis: Material: skin lesion biopsy specimens:
- Microscopic examination:
- Staining procedures – Giemsa stain
- Cultivation technique: cultivation in Novy-MacNeal-Nicolle (NNN) medium
Cutaneous amebiasis. Material: bipsy specimen from skin lesion:
- Microscopic examination:
- Staining procedures – trichrome,
- Examination of histological material
- Cultivation technique: cultivation in Pavlova’s medium
Toxoplasmosis. Materials: biopsy specimens - lymph node, spleen, liver, intraocular fluid, placenta, amniotic fluid, as well as cerebrospinal fluid and serum for immunodiagnosis:
- Microscopic examination:
- Staining procedures – Giemsa stain
- Examination of histological materials
- Immunodiagnostics: ELISA (IgG, IgM, IgA antibodies, ELISA – IgG avidity), Western blot
- Intraperitoneal inoculation into mice
- Detection of parasite DNA by PCR
Trypanosomiases (American and African). Materials: venous blood, biopsy material, cerebrospinal fluid
- Microscopic examination:
- Staining procedures – Giemsa stain
- Examination of histological materials
Extraintestinal amebiasis. Material: serum, skin lesion biopsy specimens
- Immunodiagnostics: ELISA, IFA
Naegleria and Acanthamoeba infections. Materials: cerebrospinal fluid, biopsy specimens (brain tissue, skin, cornea) or of corneal scrapings
- Microscopic examination:
- Wet mount preparations
- Staining procedures - trichrome, Heidenhain, Lawless stains
- Examination of histological materials
- Cultivation technique – non-nutrient agar (NNA) plates, PPG medium
- Моlecular methods - PCR
Pneumocystosis. Materials: induced sputum, bronchoalveolar lavage, biopsy specimen
- Microscopic examination:
- Staining procedures – Giemsa, toluidine blue
Еchinococcosis. Materials: serum sample, pathological specimens for detection of present scolexes in the cyst and vitality of the parasitic cyst:
- Microscopic examination:
- Examination for presence of parasitic elements – hooks, scolexes and membranes
- Methylene-blue staining for vitality determination
- Immunodiagnostics: ELISA, Western blot
Toxocariasis (Toxocarosis). Material: serum sample:
- Immunodiagnostics: ELISA, Western blot
Тrichinellosis (Trichinosis). Materials: serum sample, muscle biopsy specimen, suspicious meat products for sanitary examination:
- Microscopic examination:
- Examination for presence of trichinella larvae
- Immunodiagnostics – IHA, ELISA, Western blot
- Molecular analysis and Trichinella genotype determination – Multiplex and Nested PCR
- Sanitary examination for presence of Trichinella larvae in meat products
- Compressive trichinelloscopy (Squash preparation)
- Digestion with artificial gastric juice
Cysticercosis. Materials: serum sample, cerebrospinal fluid (CSF), and biopsy histological specimens
- Microscopic examination:
- Examination of hystological specimens for parasitic elements
- Immunodiagnostics - ELISA
Paragonimiasis. Materials: bronchoalveolar lavage, tracheobronchial secretion, induced sputum, biopsy specimen, stool
- Microscopic examination:
- Wet mount preparations
- Concentration techniques
Filariases. Materials: venous blood, biopsy specimen
- Microscopic examination:
- Staining procedures – Giemsa stain
- Concentration techniques: 2% formalin (Knott's technique)
- Examination of subcutaneous nodes (Onchocerca volvulus and Mansonella streptocerca)
- Rapid diagnostic test
Dirofilariases. Material: biopsy specimen
- Мicroscopic examination:
- Examination of histological specimens
- Маcroscopic examination, after parasite extraction
4. Urogenital parasitic diseases.
Тrichomonasis. Materials: urine, semen, prostate exprimate, vaginal and cervical swab specimens, urethral swab specimen:
- Microscopic examination:
- Wet mount preparations
- Staining procedures – Giemsa stain
- Cultivation techniques: cultivation in TV4 medium
- PCR
Schistosomiasis (urogenital). Materials: 24 hours urine, urine collected in the interval between 11.00 and 15.00 o’clock – after physical exercise, bladder biopsy specimen:
- Microscopic examination:
- Wet mount preparations
- Concentration techniques – sedimentation, formalin - ethyl acetate sedimentation technique
- Rapid diagnostic test – with urine
Members of the laboratory participate in the implementation of the following scientific projects financed by the Scientific Research Fund:
Project title: "Molecular genetic studies on the prevalence of human Pneumocystis pneumonia in Bulgaria", project no. KP-06-N33/18 dated 21.12.2019
Duration: 36 months
Project leader: assoc. prof., PhD, Nina Dimitrova Tsvetkova
Pneumocystosis is an opportunistic infection that in immunocompromised individuals, elderly, and young children can lead to serious complications and even death. Early and timely diagnosis, the conduct and monitoring of etiologic treatment are critical to the outcome of the disease.
The aim of the project is to investigate the prevalence of pneumocystosis in different groups of immunocompromised and immunocompetent individuals, to identify the levels of colonization/carrier of the pathogen among at-risk populations, and to clarify the role of colonized individuals as a possible reservoir for the spread of P. jirovecii infection.
The introduction of molecular genetic methods in the diagnosis of pneumocystosis will have a positive effect in several directions, the more important of which are: a) improving the quality of diagnosis of this disease; b) monitoring of the results of conducted treatment by quantifying the pathogen in the clinical material under study; c) accumulation of new data on the epidemiology of P. jirovecii in Bulgaria - to the best of our knowledge, no such studies have been done in the country so far.
In our country the data on the incidence of pneumocystosis need to be updated, expanded, and deepened. This project proposal will be the first study to cover a broad contingent of different patient risk groups to be tested for Pneumocystis pneumonia and colonization/carrier of the pathogen by a combination of diagnostic methods including advanced highly sensitive molecular genetic methods, which will increase the detectability of infected persons.
The use of PCR techniques to demonstrate colonization/carrier of P. jirovecii in clinically healthy individuals (medical staff, relatives or caregivers) coming into contact with patients diagnosed with Pneumocystis pneumonia will lead to increased pathogen detectability due to the potential of molecular genetic techniques to detect a very small number of microorganisms in the analyzed samples, respectively the clinical materials.
The study on gene coding for dihydropteroate synthase (DHPS), which is the target area in the mechanism of action of sulfones and sulfonamides, by restriction fragment length polymorphism (RFLP) analysis will allow new data on the development/lack of resistance of P. jirovecii strains to be obtained and to evaluate the risk of spreading P. jirovecii strains with DHPS gene mutations. This will result in the accumulation of new original data on the circulating strains in our country.
Research methodology and techniques:
Clinical materials (sputum, tracheal aspirate, bronchoalveolar lavage material, mouthwashes, etc. - the type of material depends on the patient's condition) will be tested with the following methods:
1 Standard staining methods for direct microscopy
a) Azure-eosin-methylene blue staining, Romanowsky-Gimza method,
b) Toluidine blue staining (selective method for P. jirovecii cysts),
c) Gomori Methenamine-silver nitrate staining (for P. jirovecii cysts).
2 Molecular genetic techniques
Genomic DNA extraction.
Real-time PCR for detection of P. jirovecii.
PCR-RFLP for detection of point mutations in dihydropteroate synthetase (DHPS) gene.
- Project title: "Studies on the cellular and humoral immune response in patients with acute and chronic form of toxoplasmosis", KP-06-N43/2 of 27.11.2020.
Duration: 36 months
Project leader: Prof. Iskra Raynova, MD, DSc
- Project title: "Biological and molecular genetic studies on the insecticide resistance of native and invasive mosquito species", KP-06-N41/5 dated 30.11.2020.
Duration: 36 months
Project leader: Assistant Professor Ognyan Mikov, PhD
- Project title: "Phylogenetic and genotypic analysis of Enterobius vermicularis and studies on the influence of the parasite on the local immunity of the intestinal tract and the possibility of bacterial co-infections in infected persons in Bulgaria", KP-06-PN53/4 of 11.11.2021.
Duration: 36 months
Project leader: Assistant Professor Eleonora Kaneva, PhD
Written by Super UserDepartment of Parasitology and Tropical Medicine

Prof. Iskra Rainova, MD, D.Sci.
In 1958 activities and opportunities of NCIPD significantly enrich with the formation of Parasitology Department at the Centre, on the basis of the existing Republican Institute of Malaria and Medical Parasitology. Department of Parasitology and Tropical Medicine has made significant contributions in the surveillance and control of parasitic and tropical diseases. The staff of the Department is composed of perfectly trained professionals who develop policy and practical approaches in this important field for the country.

The main activities carried out in the Department of Parasitology and Tropical Medicine are focused in several directions:
1. SCIENTIFIC RESEARCH. The epidemiology, immunology, pathogenesis, clinic, diagnosis, treatment and prophylaxis of indigenous and imported parasitic diseases are studied.
- The efforts are mainly dedicated to the widely spread indigenous parasitoses such as zoonoses - echinococcosis, trichinellosis, taeniasis; soil-transmitted helminthoses - ascariasis, trichuriasis, toxocarosis; cmmunity acquired parasitic diseases - hymenolepiasis, enterobiasis, giardiasis.and trichomoniasis.
- Opportunistic parasitoses (toxoplasmosis, cryptosporidiosis, blastocystosis, visceral leishmanisis) in immunocompromised patients are diagnosed by morphological, serological and biomolecular methods. Ethiological structure, clinical peculiarities and epidemiology of these infections are investigated.
- The imported parasitic diseases – malaria, amoebiasis, filariases, ancylostomidoses, leishmaniases, etc. are mainly studied in order to restrict the consequenses of their import, to prevent their local transmission and epidemic and endemic distribution in the country.
- The development and implementation of control measures programs concerning parasitic diseases are also on agenda. Under the leadership of the MH the staff of the department has developed two National Programs: between 2004 and 2008 the program for control of echinococcosis in humans and animals and from 2014 until 2018 National Program f of vector-borne transmitted infections in humans in Bulgaria.
- The problems of ecoparasitology are the topic of a research work, too. Naegleria and Acanthamoeba spp. are studied. An entomology unit is operating at the department, dealing with the blood-sucking insects of actual or potential importance as vectors of parasitic diseases.
2. TRAINING ACTIVITIES. As a part of NCIPD the department is accredited by the Ministry of Education and Research as a National Postgraduate Training Centre in the basic medical specialty – medical parasitology for continuous education and PhD program “Parasitology and Helminthology”. There is an annual program of short- and long-term postgraduated training courses and individual training.
3. Diagnostic and therapeutic activitIES. Routine and reference diagnosis of indigenous and imported parasitic diseases is carried out by modern laboratory methods - morphological, immunological and molecular – biological. Medical consideration of patients, incl. travelers is conducted.
4. ADVISING AND CONSULTING ACTIVITIES. The staff is engaged in epidemiological surveillance of parasitic diseases in the country, in the development of annual analysis of the parasitological situation, in elaborating standard documents and national programs for the control and prevention of parasitic diseases in the country in assistance to the Ministry of Health. Technical assistance to the specialized parasitological network, to the clinicians of other specialties and to the general practitioners in all aspects of parasitology – epidemiology, diagnosis, treatment, prophylaxis, control is carried out as well.
5. External Quality Assessments (EQA). From 2001 NCIPD and Department of Parasitology and Tropical Medicine conducted EQA of laboratory diagnostics for all parasitological laboratories in Bulgaria and from 2013 certified several national malaria laboratories in other countries of the WHO European Region. Every year NCIPD and the Department participate in external laboratory quality control of diagnostic activity organized by INSTAND, Germany.
The Department includes the following main units: National Reference Laboratory for the Diagnosis of Parasitic Diseases (NRLDPD), Laboratory of Experimental and Applied Parasitology (LEPP) and National Outpatient Unit for Consulting Assistance in Parasitic and Tropical Diseases.
Main publications in journals in recent years:
Harizanov, R., I. Rainova, N. Tzvetkova, I. Kaftandjiev, I. Bikov, O. Mikov. Geographical distribution and epidemiological characteristics of visceral leishmaniasis in Bulgaria, 1988 to 2012. // Eurosurveillance, 18, 2013, №29, 7-12. IF 5,49
Harizanov, R., I. Rainova, I. Kaftandjiev, D. Jordanova, I. Marinova. Soil-transmitted helminth infections in Bulgaria. A retrospective study of some epidemiological features. // Problems Inf. Parasit. Dis. 41, 2013, 2, 32-35.
Rainova, I., I. Marinova, R. Harizanov, D. Jordanova, I. Kaftandjiev, I. Bikov, N. Tsvetkova. Parasitic diseases in Bulgaria in 2012. // Problems Inf. Parasit. Dis. 42, 2014, 1, 29-38.
Harizanov, R., D. Jordanova, I. Bikov. Some aspects of the epidemiology, clinical manifestations, and diagnosis of human dirofilariasis caused by Dirofilaria repens. // Parasitology Research, 113, 2014, 4, 1571-1579. IF 2,327
Gottstein B., J. Wang, G. Boubaker, I. Marinova, M. Spiliotis, N. Muller, A. Hemphill. Susceptibility versus resistance in alveolar echinococcosis (larval infection with Echinococcus multilocularis). // Vet. Parasitol. 213, 2015, 3-4, 103-109. (IF= 2,460)
Jordanova D. P., R. N. Harizanov, I. T. Kaftandjiev, I. G. Rainova, T. V. Kantardjiev. Cystic echinococcosis in Bulgaria 1996–2013, with emphasis on childhood infections. // Eur J Clin Microbiol Inf Dis, 34, 2015, 7, 1423-1428. (IF 2,668)
Kaneva E., I. Rainova, R. Harizanov, G. Nikolov, I. Kaftandjiev, I. Mineva. Study of Toxocara seroprevalence among patients with allergy and healthy individuals in Bulgaria. // Parasite Immunology, 37, 2015, 10, 505-509. (IF 2,143)
Kaftandjiev I. T., R. N. Harizanov. Rare case of epididymal dirofilariasis. // QJM: An International Journal of Medicine, 109, 2016, 5, 351-352. (IF 2,824)
Muhtarov M., I. Rainova, F. Tamarozzi. Treatment of hepatic cystic echinococcosis in patients from the southeastern Rhodope region of Bulgaria in 2004-2013: Comparison of current practices with expert recommendations. // American Journal of Tropical Medicine and Hygiene, 94, 2016, 4, 900-905. (IF 2,453)
Rainova I, I. Kaftandjiev, R. Harizanov, N. Tsvetkova, D. Jordanova, I. Marinova, R. Kurdova, T. Kantardjiev, N. Lalkovski. Outbreaks of human trichinellosis, still a challenge for the public health authorities in Bulgaria. // Journal of Public Health, 24, 2016, 4, 291-297. (IF 2,06)
Yancheva N., D. Strashimirov, I. Elenkov, T. Tchervenyakova, Р. Tomova, N. Tcvetkova, M. Yankova, T. Tomov, M. Nikolova. Etiologic characteristics of enterocolitis in hospitalized HIV-infected patients for 3-year period (2013-2015). // Acta Medica Bulgarica, XLIII, 2016, 1, 64-70.
Yancheva N, N. Tsvetkova, M. Nikolova, I. Alexiev, T. Tchervenyakova. HIV-Infected Patient with Refractory Giardiasis and Lingua Villosa Nigra: A Case Report. // Clinical Medical Reviews and Case Reports, 3, 2016, 8, 126.
Written by Super User

 English
English
 Bulgarian
Bulgarian 